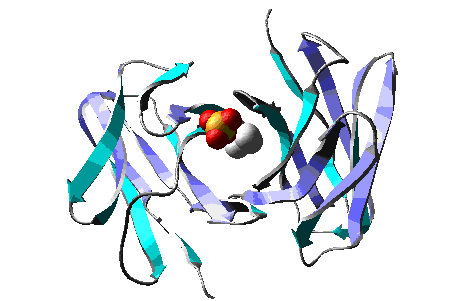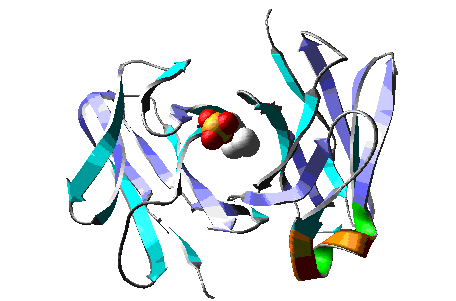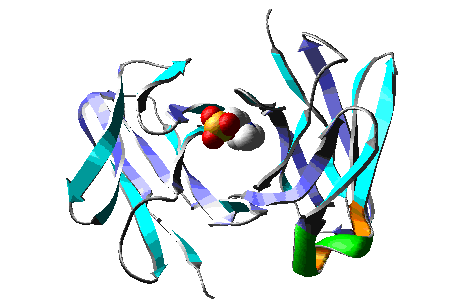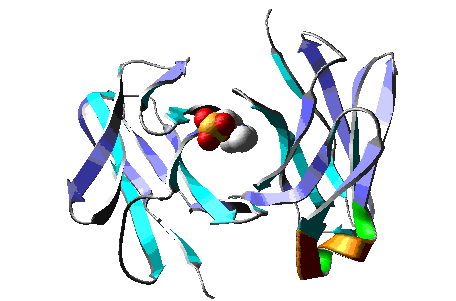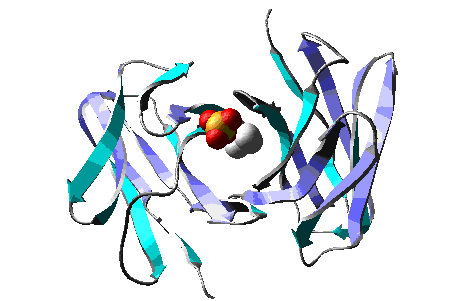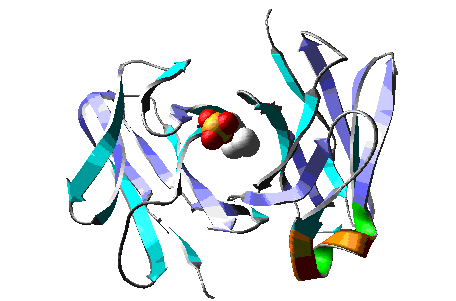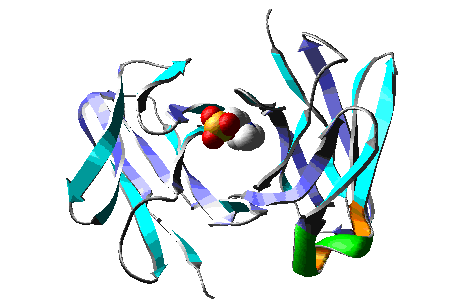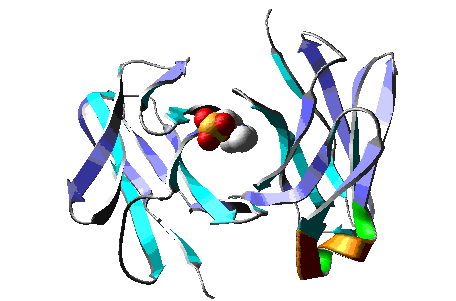Antibody-Antigen Binding
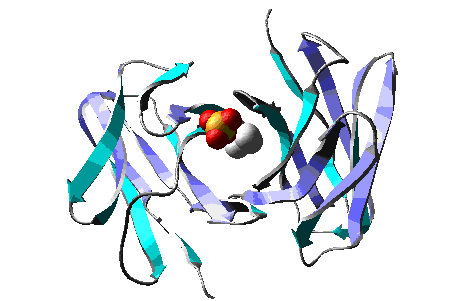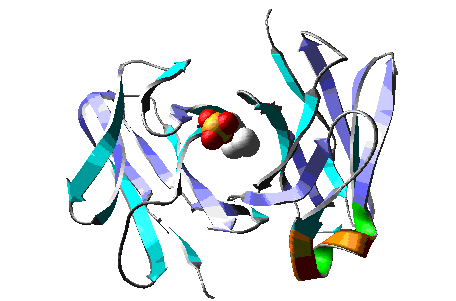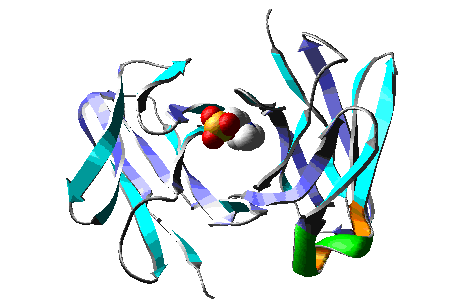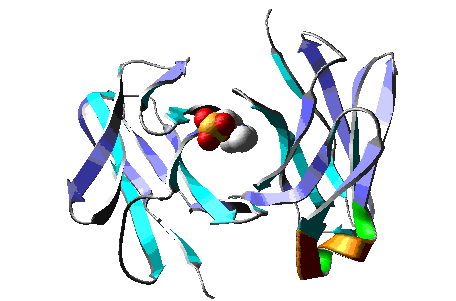 |
Molecular Dynamics simulation of a model of the antibody
McPC603 complexed with PC (phosphocholine), during > 15 ps.
More animations!
CPK
Wireframe |
We are studying the structural interactions between
antigens (or haptens) and antibodies, primarily by using nuclear magnetic
resonance (NMR) spectroscopy. The monoclonal antibodies (mAbs)
are from the immune memory response to phosphocholine-protein conjugates
(PC-protein). This system was chosen for study because the haptens
are small and chemically distinct from the protein carrier, and because
these antibodies are sufficiently characterized enough to allow us to study
the consequences of immune maturation from a structural/energetic point
of view.
Aim 1. To evaluate specify the hapten-antibody interactions that give
rise to enhanced binding in the memory response.
This part of the project gives information about the structural reasons
for changes in antigen binding, especially within the complementarily determining
regions (CDRs) of the antibody, during immune maturation.
Aim 2. To define the contribution of the hapten phenyl ring in antibody
binding of phenylphosphocholine required by antibodies in the memory response.
The defining characteristic of "Group II antibodies" from the response
to PC-protein is the requirement of a haptenic phenyl ring.
Aim 3. To elucidate the contribution of the carrier protein in PC-protein•mAb
complexes.
We are studying the effects of different carrier protein models in
an effort to provide a systematic study of the contribution the carrier
protein to antibody affinity, both by affinity measurements and by structural
characterizations.
Immune maturation results in a changed antibody population that has
substantial changes in both affinity and selectivity. Changes in
affinity and selectivity provide the response to a broadening array of
potentially harmful antigenic structures which, in the case of microorganisms,
can diversify. Somatic mutation and clonal recruitment are the two
basic mechanisms that play dominant roles in producing antibody diversity.
The immune response to phosphocholine-keyhole limpet hemocyanin (PC-KLH)
provides a unique model to study the interrelationships between epitope
recognition and the evolution of specificity in the initial and memory
responses.
Two distinct antibody populations (Groups I & II) respond to PC-KLH.
The distinction between Groups I and II antibodies was originally based
on the observation that binding to PC-protein by antibodies in the primary
response can be blocked by PC, while binding to PC-protein by IgG antibodies
in the memory response cannot be blocked by PC; this binding is blocked
by p-nitrophenylphosphocholine. These antibodies from the memory
response were called Group II, and the PC-reactive antibodies from the
primary response were called Group I. Other groups recognized the
presence of Group II antibodies. The Group II population has also
been subdivided into two sub-groups, based on the ability to bind NPPC
analogs: Group IIA antibodies require the positively-charged choline
nitrogen for binding, while Group IIB antibodies can also p-nitrophenyl-3,3-dimethylbutylphosphate
(NPDBP), which lacks the positive charge. Therefore, various regions
of the PC-protein epitope contribute, in different degrees, to binding
to various Group II antibodies. For us, this means that the immune
response to PC-KLH can be used to evaluate the importance of the variations
in epitope recognition and affinity that determine the memory B cell pool
composition.
Return to David Peyton's home page