Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2007 |
Quiz 10 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2007 |
Quiz 10 |
![]()
1. (3 points) Write the expected products for each of the following reactions:
a) 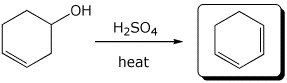
b) 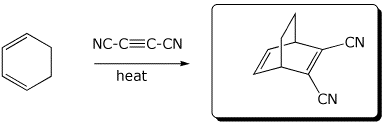
c) 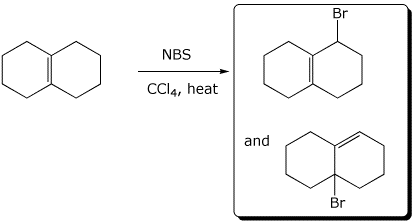
2. (2 points) Draw clear pictures that show the orbital symmetry of the HOMO and the LUMO for s-trans-1,3-butadiene.
3. (5 points) Addition of DI to the diene below gives two allylic cations (A and B), which give two products each (W, X, Y, and Z).
Ignoring stereochemistry,
write structures for the two resonance forms each for both cations A
and B,
and write structures for the four products
W, X, Y, and Z.
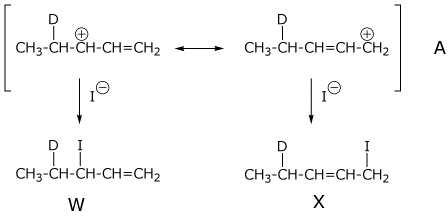
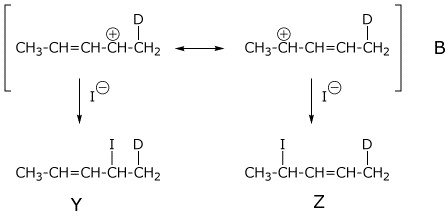
Which cation is likely to be more stable? A or B (circle one letter).
(both ends of the allylic cation are 2°)
Including stereochemistry, how many stereoisomers are possible for each of the four products?
W 4
X 4
Y 4
Z 4