Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2005 |
Exam 3 Answer Key |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2005 |
Exam 3 Answer Key |
![]()
1. (15 points) Write complete names for each of the following, including stereochemistry if it is specifically shown.
a) ![]()
p-methylbenzyl magnesium bromide
b) ![]()
4-t-butoxy-4-methylheptane
c) ![]()
(1R,3S)-3-ethylthiocyclopentanol
d,e) give two names
(S)-2-methyl-2-propyloxirane
(S)-2-methyl-1,2-epoxypentane
(S)-2-methyl-1-pentene oxide
2. (15 points) Compare each of the following with respect to the property indicated. Write “MOST” and “LEAST” under the compounds with the highest and lowest values of the property indicated.
a) boiling point
LEAST / / MOST / / MIDDLE
b) solubility in water
MIDDLE / / LEAST / / MOST
c) acidity
MIDDLE / / MOST / / LEAST
d) reactivity towards LiAlH4
MOST / / MIDDLE / / LEAST
e) nucleophilicity
MOST / / MIDDLE / / LEAST
3. (15 points) Complete each of the following reactions by adding the missing part: either the necessary reagents and conditions or the expected major product.
a,b) 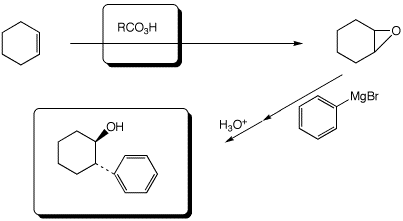
c) 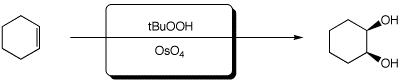
d) 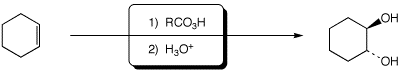
e) 
4. (15 points) Complete each of the following reactions by adding the expected major product.
a) 
b) 
c) 
d) 
e)  (ferrocene)
(ferrocene)
5. (10 points) Balance the following reaction. Chromium ends up as Cr+3 .
6. (10 points) Write complete mechanisms for each of the following reactions.
7. (10 points) Show a sequence of reactions that could be used to synthesize the compound shown below. Show all steps and the product of each step. You must start with styrene as the only source of carbon.
8. (10 points) Show a sequence of reactions that could be used to synthesize the compound shown below. Show all steps and the product of each step. You may start with any alcohol having three carbons or fewer.